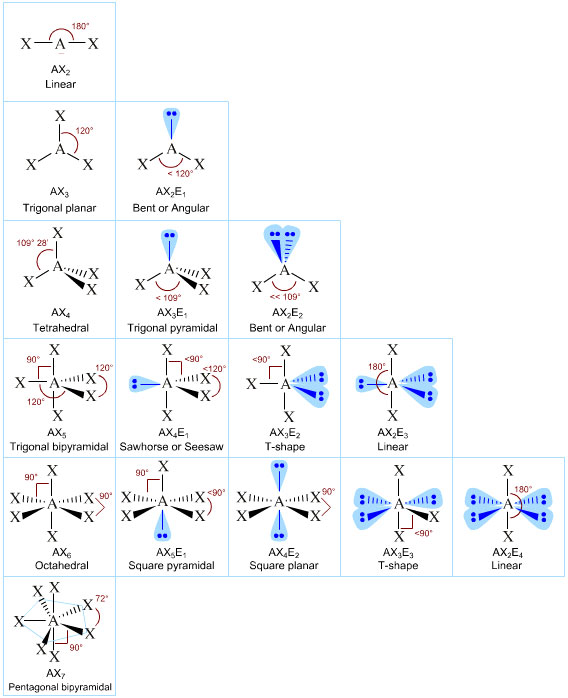
American General Chemistry textbooks – but for some reasom not British ones – adopt the excellent AXmEn system, where A is the central atom, m the number of ligands X, and n the number of nonbonded lone-pairs of electrons, E, about the central atom.
In this system:
methane, CH4, is AX4
ammonia, H3N:, is AX3E1
water, H2O, is AX2E2
Note that different AXmEn designations can give rise to the same overall geometry or shape:
For example:AX2E1 and AX2E2 both give rise to bent or angular geometries
AX2 and AX2E3 both give rise to linear geometries
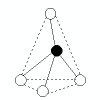
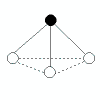
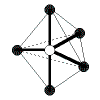
Patterns in AXE Space
The AXE system gives rise to a pattern, from which the various atomic geometric shapes can be determined/assigned: